|
||||
 |
||||
|
UV Filters Do Not Stop Fading, They Help Reduce Fading... Visible Light is produced within the spectrum of electromagnetic energy that includes radio waves, microwaves, X-ray and ultraviolet (UV) rays. The cause of fading is due to a photochemical reaction involving UV and visible light.
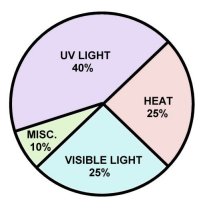
Research has shown (above) that 40% of fading is caused by UV rays. Another 25% of fading is due to heat, with 25% being caused by normal visible light. The remaining 10% cause of fading is from indoor artificial lighting, humidity, and poor dye anchorage. Visible light and UV radiation cause fading and a lack of moisture, especially in the cloth materials used to cover books.
Electromagnetic energy is measured in nanometers (nm.) UV is present from 100-400nm, and visible light is present from 400-700nm. The energy that exists from 100-700nm is thererfore responsible for 65% of all fading. These wavelengths are very damaging to library materials, dyes, papers, wood, paintings, photographs, and even the foods we eat.
Due to newer pollution control laws, the dyes used in fabrics, paints, wood stains and coatings available in home improvement centers have been formulated to have a minimal impact on our environment. Unfortunately, some are of these materials are less stable than the dyes, paints, stains and coatings that have been used in the past. That is because the older materials were usually solvent-based. While the water-based products we use today are more environmentally-friendly, they are more vulnerable to fading in time. Effect of UV Radiation on Colored Textiles Textiles include items such as clothing, quilts, embroideries, draperies, upholstery, linens, and carpets. They are an intimate part of our daily lives and are often valued for this reason. Textiles are composed of a wide variety of materials, all of which are sensitive to environmental factors such as light, humidity, temperature, and airborne soil. Protecting textiles from environmental extremes is key to their long-term preservation. Safe handling, storage, and display practices will significantly slow deterioration and prevent damage. UV rays (found either in sunlight or artificial light such as fluorescents) act as a bleaching agent. Through a complex process, UV rays transform the water found in all fabrics into hydrogen peroxide, (a common bleaching agent) that leads to the fading of dyestuffs. High energy photons of light, typically found in the ultraviolet or violet spectrum, can disrupt the bonds in the chromophore (a chromophore is the part of a molecule that is responsible for its color), leaving the resulting material colorless. Extended exposure to UV and visible light often leads to widespread discoloration. Ultraviolet light is the invisible high-energy portion of the spectrum. It is capable of causing the greatest amount of damage within the shortest period of time. People in the clothing, drapery, carpeting, upholstery and other textile related industries know that after fabrics are exposed to fluorescent lighting for a period of time, the color dyes used in fabrics fade. The photodamage to wool, for example, has serious economic implications in large producer countries. Exposure of wool keratins to sunlight is well known to cause yellowing, bleaching, and main-chain scission of the proteins. Visible radiation in sunlight causes photobleaching of wool while the UV wavelength causes photoyellowing. The most effective yellowing wavelengths were in the UV-A region (340 -420 nm). Effect of UV Radiation on Plastics UV energy absorbed by plastics can excite photons, which then create free radicals. While some plastics cannot absorb UV radiation, catalystic residues and other impurities will often act as receptors that lead to degradation. Small amounts of these impurities are sufficient for the degradation to occur, which in the case of Polycarbonate will initiate color instability. In the presence of oxygen the free radicals form oxygen hydroperoxides will lead to a brittle structure. This process is often called photo-oxidation. As an example, a polyvinyl chloride (PVC), window frame exposed to sunlight undergoes discoloration, loss of impact strength, chalking, and a reduction in tensile properties in addition to other chemical changes. Common synthetic polymers, in addition to PVC, which may be affected by UV include:
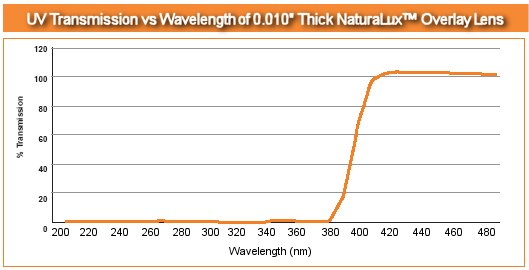
NaturaLux™ Filters absorb 100% of the harmful UV rays up to 380nm. That means all UVB and almost all of the UVA is absorbed as well. 81-99% of the UVA found between 380-390nm is absorbed. From 390-400nm, there is an 50-80% UVA absorption rate. This means that your overall exposure to UV from fluorescent lighting is practically non-existent. NaturaLux™ Filters are designed to reduce the fading process by absorbing the UV and visible light rays that ultimately damages your valuable possessions.
|
||||